Genevoyager Bac/Sf9 system leverages proprietary gene recombination technology to develop recombinant baculoviruses carrying target genes. These viruses efficiently infect insect cell lines, enabling stable and scalable production. This approach effectively addresses key challenges in large-scale vaccine manufacturing, including virus seed passage stability and batch-to-batch consistency.
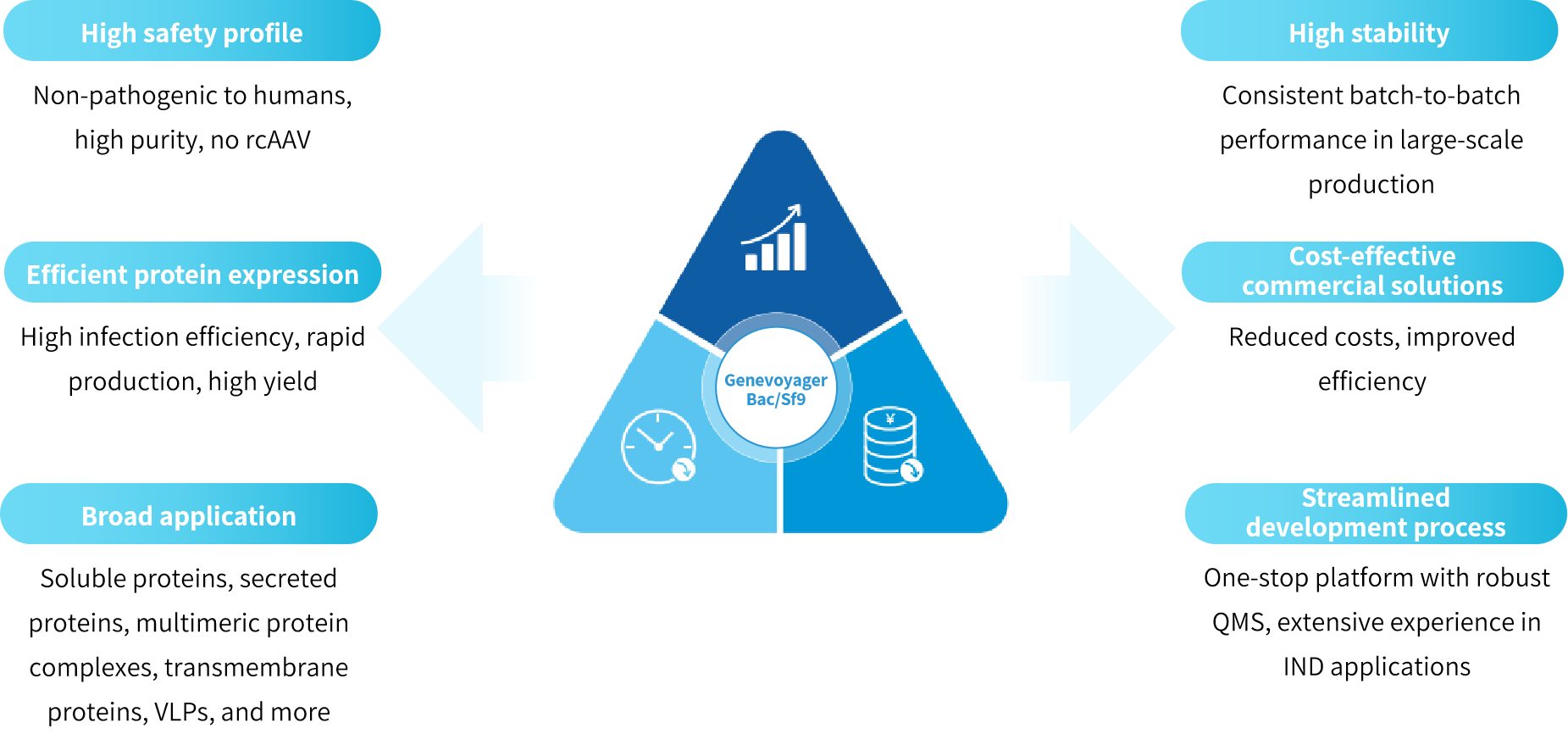
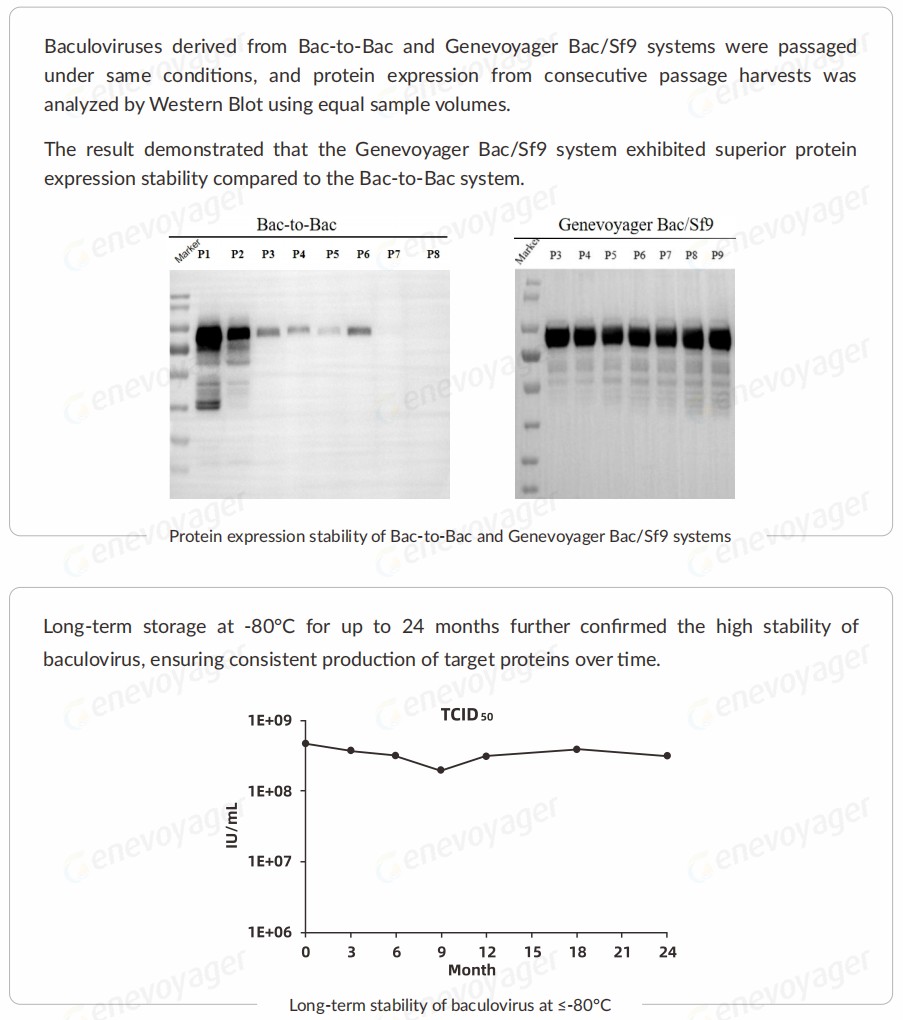
The Genevoyager proprietary Bac/Sf9 system demonstrates superior baculovirus stability, ensuring consistent immunogenicity and batch-to-batch consistency in vaccine production. The system enhances recombinant baculovirus generation, reduces protein degradation, and improves protein yields with minimal heterogeneity.
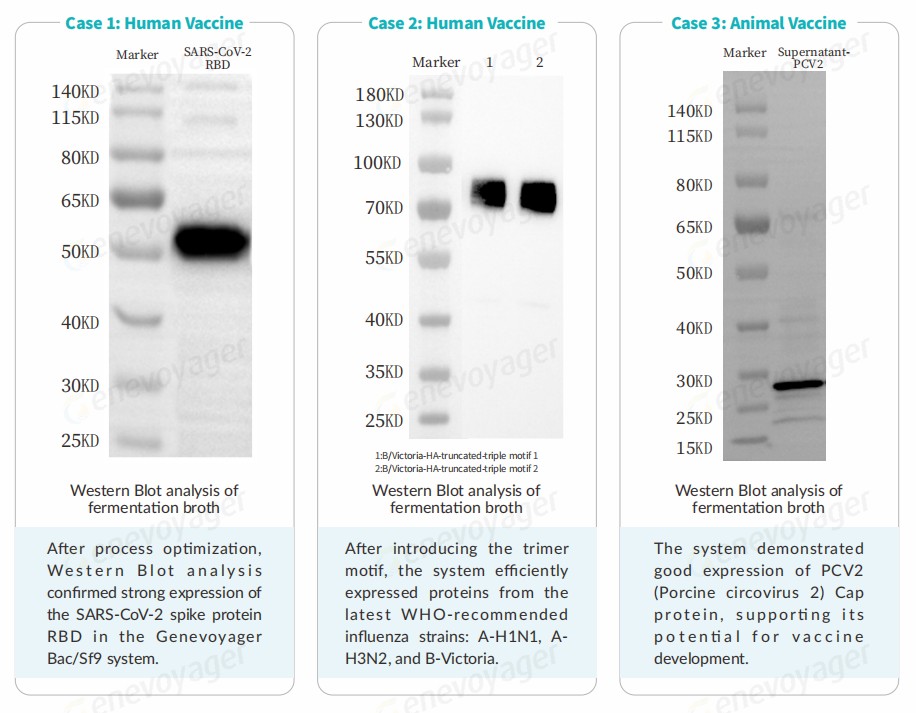
Case Studies
The Genevoyager Bac/Sf9 system has been tested in the production of human vaccines and animal vaccines, demonstrating high yield, rapid expression and scalability.
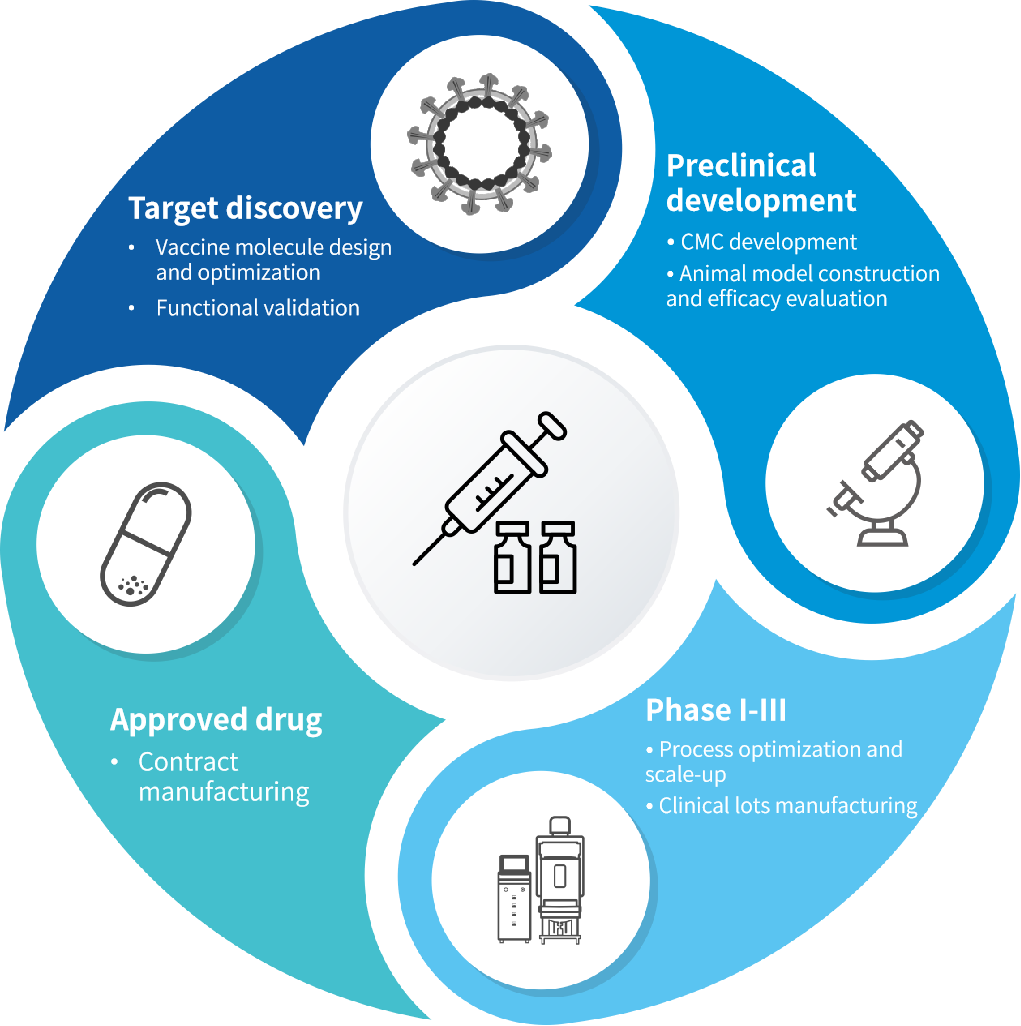
Contract Manufacturing Solutions
Genevoyager Bac/Sf9 system
● Genevoyager Bac/Sf9 provides a robust, cost-effective, and scalable solution for large-scale therapeutic and preventive vaccine production (up to 2000L) and allows for rapid adaptation to emerging pathogens.
● This system has been successfully applied in 200L GMP vaccine production, demonstrating its advantages in efficient expression of complex proteins, consistent batch-to-batch performance, and high immunogenicity.

US: 3675 Market Street, Suite 200, Philadelphia, PA19104 Tel: +1 (215) 205-6963 | +086 027-65023363
E-mail: hui.wang@genevoyager.com / vector@genevoyager.com
China: No128, Guanggu 7th Rd, East Lake High-tech Development Zone, Wuhan, China Tel: 17720522078
E-mail: marketing@genevoyager.com